| Home Page | Contents | Index | Comments? |
| Back - Astrometric project |
Portions extracted from: Investigation of ferroelectric effects in two sulfide deposits, Journal of Applied Geophysics, vol. 32, p. 55-72, 1994
Ore minerals are fundamental to civilization. They are also increasingly more difficult to find. The surface, oxide-enriched (supergene) ore bodies have mainly been found and are, or are being mined out. So today's mineral explorationist is generally seeking low-grade deposits that tend to be associated with sulfide ore minerals. As you might imagine, the physical properties of these minerals have been intensively studied since the beginnings of geology as a science.
The most common ore mineral that people see is iron sulfide, FeS 2 , or fool's gold. Another very common ore mineral is pyrrhotite, another iron sulfide, Fe 1-x S . In doing geophysical exploration for ore bodies, it is those two minerals that are most commonly found. Because they are so abundant, iron sulfides rarely have any commercial value unless some other minerals are deposited with them. For example, gold may replace some of the iron in pyrite or pyrrhotite. Other sulfides are also commonly found in association with the iron sulfides, and most of our copper, lead, zinc, cobalt, and other base metals come from such ore bodies.
Finding sulfide mineral concentrations that contain enough ore minerals to make them worthwhile to mine is a primary job of an economic geologist. However, a geologist can only see what is exposed at the surface, and drilling a hole is a very expensive proposition usually. As most of the surface deposits have been discovered, for subsurface exploration geophysical methods are now more extensively used.
One of my many jobs was as manager of geophysical research for AMAX, a large mining company, in Colorado. It has been known for nearly a century that sulfide ore minerals are good geophysical targets and are readily polarizable when an electric field is applied. One of the problems I had was that the electrical response from known ore bodies was too good and too persistent to be explained by conventional theory. That was particularly true as Zonge Engineering in Tucson and I began developing the controlled source audiomagnetotelluric method (CSAMT) in the late 1970's.
The CSAMT tool was so good that the first time we drilled on a prospect based on CSAMT I predicted ore grade mineralization between 770 m (2500 feet) and 860 m (2800 feet). Since there was very little surface expression of sulfide mineralization in what is known as a “halo,” I took considerable kidding from the geologists until the drill bit went into ore-grade molybdenite (MoS 2 ) at 755 m (2450 feet).
I will admit that the ore grade mineralization wasn't quite as thick as predicted. The drill went out of it at roughly 830 m (2700 feet). However, as proof of concept this was a spectacular success. Also, we had combined the CSAMT survey with a self potential, or spontaneous polarization (SP). Though the mineralization did not prove to be sufficient in size and grade to consider mining it, based on the geophysical surveys we were able to drill it out with five drill holes as compared to fifteen holes required to explore a similar, nearby deposit before we developed CSAMT. The savings in drilling costs alone paid for my entire research program and made believers out of a number of economic geologists.
CSAMT is now one of the most widely used exploration tools in the world.
We drilled one more hole in a very different geologic environment, this time in the Pine Nut Mountains in Nevada while I was with AMAX. There the problem was to determine the maximum depth at which sulfides could be found. As the CSAMT survey predicted a depth the bottom of the sulfides of no more than 200 m (650 feet), it was well worthwhile to drill such a shallow hole to test the known mineralization. My recollection is that the CSAMT survey was on the money on that one. Falling metal prices in the early 1980's forced the cancellation of my research program and the extractive industries, mining, oil, and gas, entered a depression worse than the Great Depression during the 1980's that lasted for nearly 20 years.
However, I was still unsatisfied with the physical mechanism underlying the electrical response we had seen in the CSAMT surveys. AMAX was kind enough to let me keep the data I had collected while we were developing the technique. But with two young children, survival was an iffy proposition during the 1980's for people with my skills. However, during this period I was looking through a dictionary for another word and happened on “ferroelectric”. Never having heard the term before I read the definition and realized immediately that this was a possible mechanism for what I was seeing in the CSAMT surveys. The question then being was whether ore minerals, particularly the common ones, were ferroelectrics.
Like any scientist, I then began a literature search to see what was already known, and to educate myself on the solid state physics of ferroelectrics. Also, during this period I became a visiting and adjunct professor at Texas A&M University. One of the graduate students there, Mark Bieniulis, had worked for me at AMAX, and I asked him if he would be interested in doing his thesis on ferroelectricity. I'm sure his arm has recovered by now and his work was very successful in demonstrating ferroelectric properties in chalcocite, Cu 2 S, one of the most common ore minerals of copper. Zonge Engineering also promoted my research with help and equipment so that I was encouraged to go on.
Laboratory studies by different investigators using various techniques have expanded the number of known ferroelectric ore minerals to about twenty at present (Table 1) from the first discovery of makedonite (PbTiO 3 ) in 1950. These include such common ore minerals as bismuthinite (Bi 2 S 3 ), cassiterite (SnO 2 ), chalcocite (Cu 2 S), pyrrhotite (Fe 1-x S), and stibnite (Sb 2 S 3 ).
It is also possible to predict other ore minerals that may be ferroelectric by extrapolation from known characteristics. Crystals with the same space group as a known ferroelectric commonly also prove to be ferroelectric. Other characteristics, such as optical anisotropy, known structural phase changes, etc., are also indicative of possible ferroelectricity. Such characteristics for individual minerals are presented in Ore Minerals, and can be found by mineral name in the index on the Zonge Engineering Web site.
Matthias (1967) pointed out that sulfur has the same high electronic polarizability as oxygen octahedra and the same propensity to form ferroelectric crystals. Thus, sulfides that are isostructural with oxide ferroelectrics may also be ferroelectric. By the same reasoning, telluride (GeTe is a well-known ferroelectric), selenide, and arsenide ore minerals are also considered to be probable or possible ferroelectrics.
By these methods, sixty-plus ore minerals that are isostructural with known ferroelectric minerals have been tabulated under Ore Minerals. With twenty known ferroelectric ore minerals and more than sixty minerals isostructural with known ferroelectrics, ferroelectricity should be regarded as a common property of ore minerals.
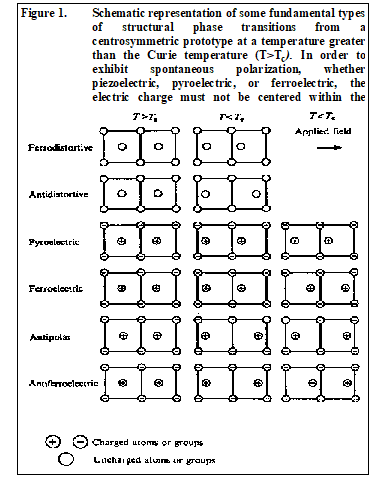
Ferroelectric materials have a spontaneous electric polarization in the absence of an applied field that can be reversed by application of a potential field. All ferroelectric crystals are simultaneously pyroelectric and piezoelectric as well. However, the converse is not necessarily true. Crystals may be piezoelectric without being pyroelectric, or piezoelectric and pyroelectric but not ferroelectric. In order to exhibit spontaneous polarization, whether piezoelectric, pyroelectric, or ferroelectric, the electric charge must not be centered within the crystal lattice.

The principle is illustrated schematically in Figure 1. Centrosymmetric crystal classes therefore are not polar dielectrics. Twenty crystal classes, of the thirty-two possible, exhibit electrical polarity, or piezoelectricity, when subjected to stress. Of the twenty piezoelectric crystal classes, ten also have a unique polar axis. These crystal classes possess a spontaneous polarization, or electric moment per unit volume that is temperature dependent, and evident from a flow of charge to and from the crystal surfaces on change of temperature. These ten crystal classes are the pyroelectric classes. If a crystal is both piezoelectric and pyroelectric, it may be ferroelectric as well if “ ...it has two or more orientational states in the absence of an electric field and can be shifted from one to another of these states by an electric field .” (Lines and Glass, 1977, p. 9). They (p. 519) relax this definition for semiconducting ferroelectrics in that “…the high electrical conductivity in all probability prevents a switching of orientational states for these systems.”
While commonly used to predict possible ferroelectricity, ferroelectricity in a crystal cannot be proven, or disproven, solely by crystallographic determination. Crystal perfection (number and type of point defects and dislocations), electrical conductivity (type and amount of impurities, extrinsic conductivity), temperature (Curie temperature and conductivity may be strongly temperature dependent functions of crystal imperfections), and pressure, may all affect the ferroelectric character of a mineral. In the absence of external stress, applied electric field, and below the Curie temperature, the polar orientation states of a ferroelectric crystal are stable with time.
Ferroelectricity is often referred to as the electrical analog of ferromagnetism.
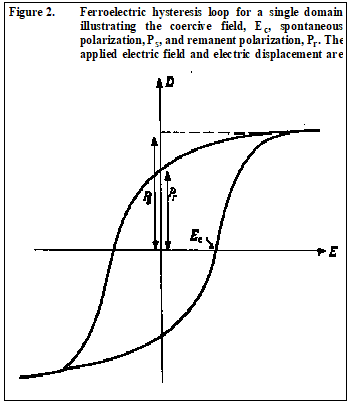
Ferroelectrics exhibit hysteresis, as illustrated in Figure 2, when polarized by an electric field and form polarization domains within a crystal in the same fashion as ferromagnetic minerals. The electrical domains are frequently characterized by twinning in the crystal. As with piezoelectric and pyroelectric minerals, ferroelectric minerals may also exhibit a surface charge.
Ferroelectrics are commonly antiferromagnetic, rarely ferromagnetic. Alexandrite, or Cr-chrysoberyl (Cr 2 BeO 4 ), is an example of a magnetoferroelectric mineral (Newnham et al., 1978).
An electrical transition from a ferroelectric state to either a paraelectric state or another ferroelectric phase occurs at some critical temperature called the Curie temperature, T c . Transition to the paraelectric state is normally associated with a phase change to a centrosymmetric crystal structure.
Dielectric permittivity (or dielectric constant) and resistivity of a ferroelectric are very nonlinear near the Curie temperature. Above the Curie temperature, the hysteresis loop (Figure 2) should ideally collapse to a straight line for the paraelectric phase of the mineral. The hysteresis is frequency dependent, and ferroelectric hysteresis can be easily confused with nonlinear lossy dielectric behavior, which is particularly troublesome with semiconductor ferroelectrics. As a result, it is very difficult, if not impossible, to obtain unambiguous hysteresis curves for semiconducting and metallic ferroelectrics, e.g., pyrrhotite.
A ferroelectric ceramic (a sulfide deposit approximates a ceramic) is defined by Lines and Glass (1977) as an aggregate of ferroelectric single crystal grains (or crystallites) with dimensions typically between 0.5 and 50 micrometers. Ceramics may have grain sizes larger than this, however, depending on the stability of individual domains within the aggregate. Ferroelectric ceramics virtually always pole, that is, they acquire a preferred directional electrical polarization of the grains when cooled through their Curie temperature. Poling can be enhanced by, or may require, application of an external electric field, stress field, or temperature gradient.
The optical frequency susceptibility of all polar crystals is anisotropic. Hence, all pyroelectric and ferroelectric crystals are optically anisotropic. In reflected light, semiconducting and metallic ferroelectric minerals exhibit bireflectance, pleochroism, and anisotropy. In ferroelectric ceramics the small individual crystals generally represent a single domain. Rotation of the ceramic under polarized light shows the preferred orientation, or poling direction, and the orientations of other domains.
Minerals may be antiferroelectric, rather than ferroelectric. For example, Ismailzade and Mirishli (1970) cite evidence that russelite (Bi 2 WO 6 ) is antiferroelectric. Other ore minerals may not prove to be ferroelectric at real temperatures, i.e., ≥ 0°K, or they are ferroelectric only at cryogenic temperatures (T c < 0°C). Altaite (PbTe) is an example of a “near” ferroelectric that is not ferroelectric at real temperatures (Lines and Glass, 1977, p. 522-524). However, with the addition of Sn or Ge to the crystal lattice the transition, or Curie temperature for altaite moves to positive (>0°K) values.
All polar dielectrics exhibit directional polarization when an external electric field is applied. Only within a single domain can the spontaneous polarization reach the saturation value ±P s (Figure 2). In a poled ferroelectric ceramic, the spontaneous polarization will be much less than saturation, thus, the poled direction will be the favorable direction for further polarization by application of an external E field.
Poling of a ferroelectric occurs when it cools through the Curie temperature, and the direction of polarization reflects the ambient stresses, temperature gradient, and external electric fields at that time. The polar direction of a ferroelectric is thus imprinted in the structure of the contained crystals and; hence, is stable with time so long as the mineral remains below its Curie temperature. Poling is the electrical analog of thermal remanent magnetization in ferromagnetic materials. Thus, directional polarization that can be amplified by application of an external electric field should be evident in ferroelectric sulfide systems. Application of an external field in other than the polar direction will still result in polarization of the body, but with reduced amplitude, as fewer electric dipole moments are favorably oriented.
Electrets include all materials in which persistent polarization is observed following the application of an electric field. Ferroelectrics are a subgroup of electrets. The relaxation times of electrets may range from seconds to years and ferroelectrics may exhibit secondary electret behavior (Parkhomenko, 1971; Lines and Glass, 1977). If an external field is applied for long time periods, domains with favorably oriented dipole moments may grow at the expense of less favorably oriented domains. On removal of the applied field, the polarization may slowly relax to its previous state. This is the electrical analog of viscous remanent magnetization.
While twenty ore minerals are presently recognized as ferroelectrics (Table 1), it is also possible to predict other ore minerals that may be ferroelectric by extrapolation from known characteristics. Crystals with the same space group as a known ferroelectric commonly prove to also be ferroelectric. New ferroelectrics are thus often discovered by looking at other crystals that have the same space group as a known ferroelectric, and other characteristics such as optical anisotropy, known structural phase changes, etc. An example of using an isostructural space group to discover a new ferroelectric mineral is given by Grigas et al. (1976) for chalcostibite, which is isostructural (space group Pna2 1 ) with bismuthinite and stibnite. Stibiotantilite is another ferroelectric mineral (Gavrilova et al., 1971) with space group Pna2 1 . By inference, bismutotantilite, dimorphite, emplectite, horobetsuite, lautite, pierrotite, and other ore minerals with space group Pna2 1 may also prove to be ferroelectric, since they are isostructural with bismuthinite, chalcostibite, stibnite, and stibiotantilite.
Matthias (1967) pointed out that sulfur has the same high electronic polarizability as oxygen octahedra and the same propensity to form ferroelectric crystals. Examples of isostructural oxide and sulfide ferroelectrics can be found (see Ore Minerals ). Thus, sulfides that are isostructural with oxide ferroelectrics may also be ferroelectric. By the same reasoning, telluride (GeTe is a well-known ferroelectric), selenide, and arsenide ore minerals are also included. Sixty-six ore minerals that are isostructural with the known ferroelectric minerals in Table 1 have been identified. With twenty known ferroelectric ore minerals and sixty-six minerals isostructural with the known ferroelectrics, ferroelectricity should be regarded as a common property of ore minerals. Given the large number of known and probable ferroelectric ore minerals, most deposits are likely to contain at least some ferroelectric minerals. Ferroelectric effects can then be expected to dominate the electrical response of any survey over a deposit containing significant quantities of ferroelectric sulfides. Based on these results, two successful field surveys were undertaken to determine if ferroelectric effects could possibly be isolated in sulfide deposits containing known ferroelectric minerals.
Two field investigations of sulfide ore bodies that contain known ferroelectric minerals were undertaken to investigate possible in-situ effects in the deposits. These deposits, at Mount Emmons (38°53×N ×107°03×W), Colorado, and Three R Canyon (31°28×N 110°46×W) in the Patagonia Mountains, Arizona, demonstrate ferroelectric effects that include directional polarization and apparent resistivity, electrically-excited resonance, and lack of reciprocity. Other phenomena include history-dependent electrical behavior and inductive effects.
Ferroelectrics polarize as a function of applied potential. It is much easier to generate a large potential than the high currents commonly used in more conventional induced polarization (IP) surveys. Thus, in electrical surveys of deposits known, or suspected, to contain ferroelectric minerals it may be advantageous to maximize the applied potential. It may also be relatively easy to induce electrical resonance in these deposits that could provide an inexpensive reconnaissance technique.
In induced polarization and complex resistivity (CR) surveys, ferroelectric effects can mask the deposit since the apparent resistivity may approach zero in the polar direction, or chargeability may be undetectable. Phase relations will most likely be uninterpretable in CR surveys over such deposits. In controlled source audiomagnetotelluric (CSAMT) surveys, directional resonance effects may preclude depth interpretation. Frequency-dependent inductive and capacitive effects may be observed in CR and CSAMT surveys over deposits containing significant quantities of ferroelectric minerals.
Ferroelectricity may also be useful in exploitation efforts for electrical beneficiation during ore processing.
Bhide, V.G., and Damle, R.V., 1960, Dielectric properties of manganese dioxide I, II: Physica, 26 , 33-42, 513-519.
Bieniulis, M.Z., Corry, C.E., and Hoskins, E.R., 1987, Ferroelectricity in natural samples of chalcocite, Cu 2 S: Geophys. Res. Lett., 14 , 135-138.
Corry, C.E., 1985, Spontaneous polarization associated with porphyry sulfide mineralization: Geophysics, 50 , 1020-1034; also see discussion in v. 51, no. 5, p. 1153-1155.
Corry, C.E., 1994, Investigation of ferroelectric effects in two sulfide deposits: Jour. Appl. Geophys., 32 , 55-72.
Corry, C.E., Carlson, N.R., and Zonge, K.L., 1988, Case histories of controlled source audio-frequency magnetotelluric surveys: 58th Ann. Internat. Mtg., Soc. Expl. Geophys., Expanded Abstracts, 415-418.
Corry, C.E., Emer, D., and Zonge, K.L., 1987, Controlled source audio-frequency magnetotelluric surveys of porphyry sulfide deposits and prospects in the cordillera of the United States: Proceedings, North American Conference on Tectonic Control of Ore Deposits and the Vertical and Horizontal Extent of Ore Systems, University of Missouri-Rolla, 204-213.
Cubiotti, G., and Geracitano, R., 1967, Ferroelectric behavior of cubic ice: Phys. Lett., 24A , 179-180.
Dengel, O., Plitz, U., and Riehl, H., 1964, Ferroelectric behavior of ice: Phys. Lett., 9 , 291-292.
Deshpande, L.V., and Bhide, V.G., 1961, Dielectric properties of SnO 2 : Nuovo Cimento, serie 10, 19 , 816-817.
Fridkin, V.M., 1980, Ferroelectric semiconductors: Consultants Bureau.
Gavrilova, N.D., Karyakina, N.F., Koptsik, V.A., and Novik, V.K., 1971, Ferroelectric behavior of the mineral stibiotantalite: Sov. Phys. Dokl., 15 , 1075-1077.
Goldsmith, G.J., 1956, Ferroelectricity in colemanite (abstract): Bull. Am. Phys. Soc., 1, 322.
Grigas, I., Mozgova, N.N., Orlyukas, A., and Samulenis, V., 1976, The phase transitions in CuSbS 2 crystals: Sov. Phys. Crystallogr., 20 , 741-742.
Hellwege, K.H., and Hellwege, A.M., 1982, Ferroelectrics and related substances, subvolume b, non-oxides: Landolt-Börnstein, New Series, Group III, 16 , Springer-Verlag.
Ismailzade, I.G., and Mirshli, F.A., 1970, High temperature X-ray and dielectric investigation of Bi 2 WO 6 : Sov. Phys. Crystallogr., 14 , 636-637.
Jones, A.G., 1983, The problem of “current channelling”; a critical review: Geophys. Surv., 6 , 79-122.
Jordan, C.E., Sullivan, G.V., Davis, B.E., and Weaver, C.P., 1980, A continuous dielectric separator for mineral beneficiation: RI 8437, U.S. Bur. Mines.
LeCorre, Y., 1957, Étude optique et diélectric de la boracite: J. Phys. Radium, 18 , 629-631.
Lines, M.E., and Glass, A.M., 1977, Principles and applications of ferroelectrics and related materials: Clarendon Press.
Litov, E., and Anderson, O.L., 1978, Possibility of ferroelectric-like phenomena in the mantle of the earth: Jour. Geophys. Res., 83 , 1692-1698.
Matthias, B.T., 1967, The search for new materials, in Weller, E.F., Ed., Ferroelectricity: Elsevier Science Publ. Co., Inc., 176-182.
Maxwell, M., Russell, R.D., Narod, B.B., and Ghomsei, M.M., 1987, Piezoelectric and electromechanical exploration methods (abstract): EOS, Trans. Am. Geophys. Un., 68 , 45.
Newnham, R.E., Kramer, J.J., Schulze, W.A., and Cross, L.E., 1978, Magnetoferrolectricity in Cr 2 BeO 4 : Jour. Appl. Phys., 49 , 6088-6091.
Nicolini, L., 1959, Ferroelectric properties of a material made of titanium oxide: Nuovo Cimento, serie 10, 13 , 257-264.
Olhoeft, G.R., 1981, Electrical properties of rocks, in Touloukian, Y.S., Judd, W.R., and Roy, R.F., Eds., Physical properties of rocks and minerals, II-2 : McGraw-Hill, 257-329.
Parkhomenko, E.I., 1971, Electricfication phenomena in rocks, trans. by G.V. Keller: Plenum Press.
Pelton, W.H., Ward, S.H., Hallof, P.G., Sill, W.R., and Nelson, P.H., 1978, Mineral discrimination and removal of inductive coupling with multifrequency IP: Geophysics, 43 , 588-609.
Rinkyavichyus, V.S., and Mikalkevichyus, M.P., 1967, Pyroelectric effect in antimony trisulfide single crystals: Sov. Phys. Solid State (English trans.), 9, 2360-2361.
Sawada, S., Nomura, S., and Fujii, S., 1958, Ferroelectricity in phase III of KNO 3 : Jour. Phys. Soc. Japan, 13 , 1549.
Schloessin, H.H., and Timco, G.W., 1977, The significance of ferroelectric phase transitions for the earth and planetary interiors: Phys. Earth Planet. Inter., 14 , P6-P12.
Shirane, G., Hoshino, S., and Suzuki, K., 1950, X-ray study of the phase transition in lead titanate: Phys. Rev., 80 , 1105-1106.
Sobolev, G.A., Demin, V.M., Narod, B.B., and Whaite, P., 1984, Tests of piezoelectric and pulsed-radio methods for quartz vein and base metal sulfides and Giant Yellowknife mine, N.W.T., and Sullivan mine, Kimberley, Canada: Geophysics, 49 , 2178-2185.
Sumner, J.S., 1976, Principles of induced polarization for geophysical exploration: Elsevier Science Publ. Co., Inc.
Thomas, J.A., and Galey, J.T., Jr., 1978, Mt. Emmons, Colorado molybdenum deposit (abstract): Am. Inst. Mining Metall. Petroleum Engineers, 107 th Ann. Mtg., Program, 44.
van den Berg, C.B., 1970, Ferroelectricity in the system Fe 1–x S: Physica Status Solidi, 40 , K65-K68.
van den Berg, C.B., 1972, Model for the semiconducting uniaxial ferroelectric Fe 1–x S: Ferroelectrics, 4 , 103-116, 195-212.
van den Berg, C.B., van Delden, J.E., and Bouman, J., 1969, α -transition in FeS – a ferroelectric transition: Physica Status Solidi, 36 , K89-K93.
White, W.H., Bookstrom, A.A., Kamilli, R.J., Ganster, M.W., Smith, R.P., Ranta, D.E., and Steininger, R.C., 1981, Character and origin of Climax-type molybdenum deposits: Economic Geology, 75 th Anniversary Volume, 270-316.
Wolfe, R.W., Newnham, R.E., and Kay, M.I., 1969, Crystal structure of Bi 2 WO 6 : Solid State Commun., 7 , 1797-1801.
Yuodvirshis, A.V., Kadavichus, V.V., and Pipinis, P.A., 1969, Electron emission, pyroelectric effect, and dielectric properties of bismuth trisulfide: Sov. Phys. Solid State, 11 , 1158-1159.
| Home Page | Contents | Index | Comments? |
| Back - Astrometric project |